New chemistry research programs aim to achieve an organic unity of student learning and development. The task of the teacher is not only to equip schoolchildren with knowledge and practical skills but also mental operations. And the student’s task is to arm himself, respectively.
One of the most important mental operations by which knowledge is acquired is comparison. Logically, the comparison is presented as the basis of generalization, on the one hand, and as the unity of such logical operations as analysis and synthesis, on the other. But in order to form a comparison as a method of their mental activity, it is necessary to use comparison as a method of training. The use of comparison as a didactic technique is an indispensable condition for the formation of analytical and synthetic activity. Students get the task of writing an essay specifically for this purpose.
The Essence of Comparison and Contrast When Writing an Essay on Chemistry
Among the thinking methods of studying chemistry, an important place is taken by comparison. The comparison process activates cognitive activity, develops abstract thinking, conscious and lasting assimilation of knowledge. This technique consists of comparing when features are distinguished by which the comparison is made, and contrast, which establishes the similarity and difference between objects.
Objects or phenomena can be compared according to one or more grounds. In the process of comparison, students study not only external signs, but also properties. A comparison helps to track objects and phenomena in change and development.
Comparing objects and phenomena with each other, students identify private and general, essential and non-essential features. A generalization is based on the identification of common and essential features. It is this that leads to the formation of concepts, to the knowledge of regular relationships and relationships.
The comparison makes it possible to identify new aspects of objects, their relationships, such features of objects and phenomena that are not perceived when studied separately. In the process of comparison, students penetrate into the essence of objects, without outside help they notice properties that are imperceptible at first glance, comprehend the features of phenomena.
Let’s Start with Something Simple
As you already understood in our chemistry assignment help, we will compare the general and distinctive features of two chemical elements, phenomena or reactions. Therefore, we need to develop an action plan that can be arranged in a table.
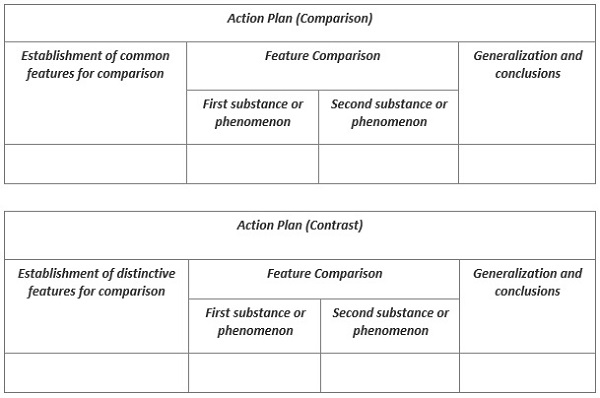
Depending on the assignment, the student makes a conclusion on the basis of incomplete or complete comparison or the most significant signs by which the studied objects are compared and contrasted.
Complications at Different Stages of the Formation of the Method of Comparison and Contrast
You can go further in your work and work to compare and contrast the signs of chemical substances or phenomena in a deeper way according to the following table.
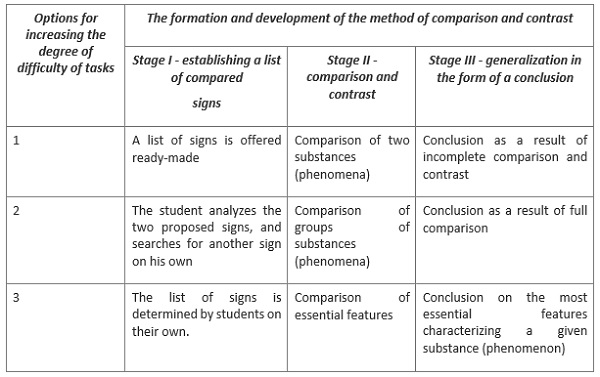
For example, according to this table, you can find several similar and different physical properties of aluminum and copper, due to which these metals find the same application.
What to Compare, What to Contrast
The subject chemistry provides rich and diverse material for comparison. We list those concepts and topics while studying, you can write a comparison and contrast essay:
- Pure Substances and Mixtures;
- Physical and Chemical Phenomena;
- Simple and Complex Substances;
- Chemical Reactions of Compound, Location, Substitution, and Exchange;
- The Main Types of Fuel and Methods for Burning Them;
- Oxides, Bases, Acids, and Salts;
- The Solubility of Substances in Water;
- Exo and Endothermic Reactions;
- Metallic and Non-Metallic Elements;
- S and P Electrons;
- Hydrolysis of Salts;
- Mechanisms of Covalent Bond Formation;
- Chemical Properties of Ammonium Salts And General Properties Of Salts. Nitrogen Oxides (II) And (IV);
- Chemical Properties of Nitric Acid and General Properties of Acids;
- Chemical Properties of Nitrates and General Properties of Salts;
- Allotropic Modifications of Phosphorus;
- General Characteristics of the Elements of the Main Subgroup of Group V;
- Allotropic Modifications of Carbon;
- Carbon Oxides (II) and (IV);
- Small and Large Periods;
- Groups and Subgroups of Chemical Elements;
- Types of Chemical Bonds;
- Types of Crystal Lattices;
- Redox Reactions and Reactions That Occur Without Changing the Degree of Oxidation of the Elements of the Substances Involved in Them;
- Comparative Characteristics of Halogens;
- Allotropic Modifications of Oxygen and Sulfur;
- Sulfur Oxides (IV) and (VI);
- General Characteristics of the Oxygen Subgroup;
- Electrolytes and Non-Electrolytes;
- Dissociation of Acids, Alkalis, and Salts;
- Strong and Weak Electrolytes;
- The Equations of Reactions in Molecular and Ionic Forms;
- General Characteristics of the Elements of the Main Subgroup of Group IV;
- Properties of Hydrogen Compounds of Non-Metals of Various Subgroups;
- Elements are Metals and Non-Metals;
- Metal Bond;
- Electrolysis;
- Corrosion of Metals and Alloys;
- Comparative Characteristics of Alkali Metals;
- Composition, Structure, Physical and Chemical Properties of Oxygen-Containing Compounds;
- Composition, Structure, Physical and Chemical Properties Of Nitrogen-Containing Compounds;
- Types of Isomerism;
- Types of Connections;
- Types of Reactions;
- Allotropic Modifications of Iron;
- Hydroxides of Iron;
- Iron Alloys Are Cast Iron and Steel;
- The Main Methods of Industrial Production of Metals and Alloys;
- Organic and Inorganic Substances;
- Types of Hybridization;
- S and P Bonds;
- Composition, Structure, Physical and Chemical Properties of Hydrocarbons;
- Methods of Refining Petroleum Products;
- Hydrogen Bond;
- Synthetic Macromolecular Compounds and Polymer Materials Based on Them.
The above concepts and topics show that comparisons can be made within one topic or section, between different topics or sections, between courses of inorganic and organic chemistry.
An Example of Comparison and Contrast in Chemistry – Structure, and Properties of Aniline Molecules
In order to correctly highlight all the necessary features, we need to write down the molecular and structural formulas of aniline and answer the question: what derivative is aniline from and what class of substances does it belong to?
It is possible to separate two components in the aniline molecule – the benzene ring and the amino group. This makes it possible to consider aniline as a derivative of benzene and ammonia and to establish that it is representative of a new class of compounds – aromatic amines.
The chemical structure of aniline allows you to compare it with the limiting amines and phenol. This comparison leads to two problems: why does aniline exhibit weaker basic properties than limiting amines, and why, in contrast to benzene, does aniline interact with bromine water under ordinary conditions?
Students already know what role hydrocarbon radicals play in enhancing the basic properties of saturated amines compared to ammonia. They are also aware of the role of the phenyl radical, which determines the weak acid properties of phenol. Comparing these two facts, first among themselves, and then in relation to the aniline molecule, students come to the conclusion that aniline, due to the influence of the phenyl radical, exhibits weaker basic properties than limiting amines.
Students establish the reason for the increased reactivity of the benzene ring in aniline by comparing it with phenol. Thus, it is possible to conclude that the amino group in the aniline molecule (like the hydroxyl group in the phenol molecule) affects the benzene core, thereby causing the substitution of hydrogen atoms under normal conditions.
How to Pick Up Research Objects for Compare & Contrast Essay in Chemistry
When comparing objects, the following requirements must be observed:
- For comparison, you should select objects that have a certain relationship with each other.
- For example, one can compare the structure and chemical properties of benzene and phenol; benzene and aniline; benzene, phenol, and aniline; structure and properties of hydrogen compounds in the period (СН4, NH3, Н2О, НF) and in the subgroup (HF, НСl, НВr, НI).
- It is necessary to clearly define the signs (properties) by which the objects are compared. So, you can compare the physical properties of metals by density, electrical conductivity, thermal conductivity, etc. The list of features should be as complete as possible, exhaustive.
- For example, to compare the spatial structure of the molecules of the initial representatives of saturated, ethylene, acetylene, and aromatic hydrocarbons, a set of attributes is distinguished: type of hybridization, valence angle, internuclear distance, the shape of the molecules.
Common Mistakes in Comparison and Contrast When Writing an Essay in Chemistry
The success of an essay depends largely on whether students have the ability to identify what is similar and different. They definitely need to learn to notice the similarities where the phenomena on the outside are very different from each other and to find a difference in those cases when the external similarity is bright.
It is a paradox that students often cannot change the way they act when completing assignments and do everything according to the template, but at the same time, they don’t apply the learned actions where necessary, because they do not know how to establish similarities.
This explains a significant part of the mistakes made in the study of chemistry and writing an essay. For example, it is difficult for students to describe the similarities and differences between neutralization reactions during the interaction of soluble and insoluble bases with acids from the point of view of the theory of electrolytic dissociation, asserting that there is no difference between them, since in both cases the essence of the reactions is to form a weakly dissociating substance – water. However, making equations of such reactions in the abbreviated ionic form helps them to establish both similarity and difference.
How to prevent such errors? How can they be eliminated if they are already approved? Practice shows that the opposite phenomena are most easily distinguished. Psychological studies prove that the contrast of concepts and rules that are different in content protects them from further confusion.
Finally – The Three Best Ways to Compare and Contrast in Chemistry
Therefore, when you need to contrast two phenomena, you can act in various ways. In the first case, two concepts (or rules) are submitted for comparison at the same time. In the second, at first one concept is studied, and then after its firm assimilation, the second is introduced as a contrast to the first. However, there is still the possibility, after sufficiently good assimilation of both concepts, to carry out their comparison.
- If all the opposite signs of the studied concepts are in sight, then you can use the simultaneous opposition, since the assimilation of the signs of one concept is reinforced by the assimilation of the signs of another. For example, it is advisable to conduct a study of the structure, properties, and application of allotropic modifications of carbon (diamond and graphite) at the same time, since you can immediately identify significant and non-essential features. So you come to the conclusion that the unequal properties of diamond and graphite are due to the different structure of their crystal lattices. In turn, their various properties determine different areas of application.
- Consistent contrast can be resorted to in cases where not all the signs of the studied concepts are pronounced. So, solid assimilation of ion exchange reactions and redox reactions is more successful if you first study the first concept, and then after a certain period of time – the second.
- And finally, the third way can be chosen in the process of generalizing knowledge of inorganic chemistry, for example, when comparing and contrasting allotropic modifications of the elements of oxygen, sulfur, carbon, phosphorus, and iron.
